BUSAN, South Korea – Meeting society’s ever-increasing energy demands while also striving for sustainability goals has become a worldwide challenge, and scientists are exploring a variety of technologies to produce clean, renewable energy that can replace fossil fuels. Besides wind and solar power, electrochemical approaches using fuel cells are attractive because they represent a compact, silent, and environmentally friendly alternative to generate electricity.
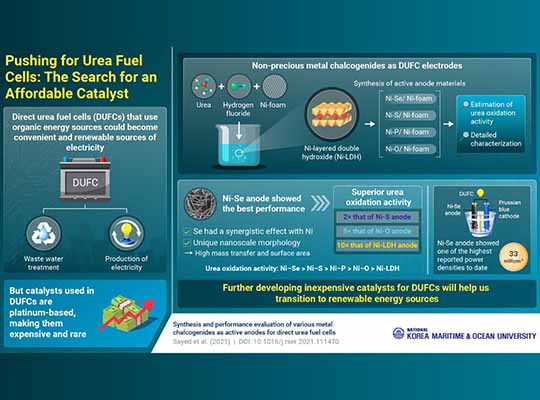
Direct urea fuel cells (DUFCs) are a particular type of fuel cell that generates electricity by breaking down urea, a nitrogen-rich molecule widely applied in fertilizers and also largely present in wastewater. However, fuel cells require catalysts to function; these are carefully selected materials that facilitate the necessary chemical reactions. In the case of DUFCs, unfortunately, the best performing catalysts known are made using precious metals, like platinum.
To tackle this limitation and find a more accessible alternative, an international research team led by Prof. Kyu-Jung Chae of Korea Maritime and Ocean University investigated a promising family of catalysts: nickel chalcogenides. The researchers used three-dimensional nickel (Ni) foam as a lightweight ‘substrate’ and then combined the outermost layer of the nickel with different chalcogen elements (oxygen, sulfur, selenium, and tellurium), producing a nanosheet of nickel chalcogenide following the complex contours of the Ni foam. They also prepared nickel phosphide (Ni–P) and nickel layered double hydroxide (Ni–LDH) in a similar fashion.
After synthesizing and characterizing the various catalysts, the team experimentally determined which one offered the best electrochemical performance, which turned out to be the one containing Ni–Se. Then, they thoroughly tested it in a real DUFC and determined what exactly gives Ni–Se an edge versus the rest, as Prof. Chae explains: “We found that selenium has a strong synergistic effect with Ni, and that the unique nanoscale morphology of the catalyst we prepared provides a high surface area to oxidize urea and enough pores to enhance mass transfer.“
The findings of this study, which was made available online on 16 July 2021 and published in volume 150 the journal Renewable and Sustainable Energy Reviews in October 2021, not only show that catalysts with different compositions can be directly grown on a lightweight support such as nickel foam, but also that nickel-based materials can be tailored to match and even outperform state-of-the-art catalysts containing precious metals. “We managed to realize a high power density in an urea-based fuel cell using inexpensive materials,” highlights Prof. Chae, “Thus, our research extends the capabilities of urea fuel cells and encourages their commercialization.“
It is worth noting that DUFCs can serve various purposes simultaneously. They can generate electricity while also helping in the treatment of urea-ridden wastewater, providing clean water in the process as well. These qualities make DUFCs a versatile option in remote places without access to a stable power grid, such as in rural areas, ships, or even space missions.
Let us hope the efforts of Prof. Chae and colleagues help pave the way to the widespread adoption of urea-based fuel cells, and on towards a brighter tomorrow.
Reference
Title of original paper: Synthesis and performance evaluation of various metal chalcogenides as active anodes for direct urea fuel cells