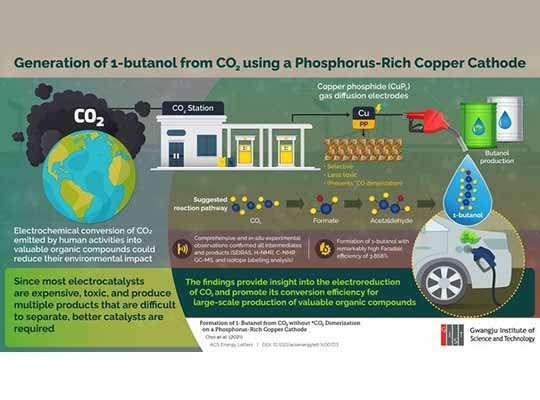
Converting CO2, which is rapidly accumulating in the atmosphere, into other valuable organic products. Now, scientists from the Gwangju Institute of Science and Technology in South Korea have proposed a reaction for the highly selective production of 1-butanol, a valuable alternative fuel, by electrochemical reduction using copper phosphide electrodes. Their findings offer a new insight on the use of Cu-based electrocatalysts for the electroreduction of CO2.
Human activities like the burning of coal and fossil fuels have caused CO2 to accumulate in the atmosphere, which has significantly affected the Earth’s climate. As a result, several scientists are looking for ways to convert CO2 into other valuable organic products, such as 1-butanol, which has shown promise as an alternative fuel for vehicles. This could help reduce our dependence on fossil fuels.
One method of obtaining useful compounds is by the electrochemical reduction reaction (CO2RR). Researchers have developed metal-based catalysts that can fulfill this task. However, there is a caveat: most of these catalysts are expensive and produce a variety of products during the reaction, which can be difficult to separate.
To solve this problem, a team of researchers led by Prof. Dr. Jaeyoung Lee and comprising Mr. Minjun Choi, Dr. Jin Won Kim, and Prof. Sungyool Bong from the Gwangju Institute of Science and Technology (GIST) in South Korea came up with a procedure that directly generates 1-butanol with the help of copper phosphide (CuP2) without first undergoing CO dimerization. “We are trying to develop a Cu-based electrode for electrochemical conversion of CO2 that avoids *CO dimerization and can help usincrease the selectivity of the product so that additional power consumption from separation processes can be avoided,” explains Mr. Minjun Choi, a Ph.D. student at the university and the paper’s first author. Their research has recently been published in volume 6 (issue 6) of the journal ACS Energy Letters on 11 June 2021 and has been available online since 11 May 2021.
Even though numerous copper-based electrocatalysts exist today, this is among the first instances in which CuP2 has been used to develop an electrocatalyst that is highly product-selective. It induces a C-C coupling reaction and circumvents the formation of CO, which is known to be a critical intermediate for Cu-based systems. The researchers confirmed this by using surface-enhanced infrared absorption spectroscopy to show that their CuP2 electrocatalyst yielded the desired product, 1-butanol, with a remarkably high Faradaic efficiency of >3%.
The team is excited about the implications of their findings. “Our goal is to design new electrodes that are stackable, that can increase production rates, and that can promote conversion efficiency so that we can make our goal of converting and using CO2 as a fuel in reality,” concludes Prof. Lee.